Overview
Until recently, the information in research publications, congress abstracts, and clinical trial data has not been readily accessible to patients and the public. PLS help to make this information easier for everyone to understand.
PLS are valuable resources for a range of audiences including patients, journalists, medical science liaison personnel, and time-poor, health care professionals.
A selection of useful resources and evidence is provided below.
“Evidence-based resources from credible sources should be easy to access and free for the people who need it. It is about time! Congratulations to all those involved in making this happen.”
Resources and Guidance

PLS of Publications Toolkit
Co-created with patients, publishers, pharma, publication professionals, and Patient Focused Medicines Development (PFMD), our evidence‑based Toolkit provides a range of resources to help you deliver effective PLS of peer‑reviewed publications and congress abstracts.

ISMPP PLS Perspectives Working Group Insights
Provides an evidence base to help inform future guidance on PLS. Explores the current challenges and opportunities for PLS using insights from diverse stakeholder groups.

Open Pharma Recommendations
Proposes minimum standards for developing short, text-only PLS published within scientific articles that can be indexed on PubMed.

PFMD ‘How-To’ Guide for co-creation of PLS
Provides a practical 7-step approach for multistakeholder co-creation of PLS of peer-reviewed publications, including journal articles and conference presentations.

Good Lay Summary Practice Guidance
Provides recommendations on how to prepare, write, translate, and disseminate lay summaries of clinical trial results. These summaries are mandated by the EU Clinical Trials Regulation No 536/2014.

Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update
PLS should be considered for all publications, as they support communication of biomedical research to many audiences, including patients.
Evidence
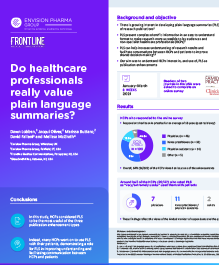
PLS are a valuable resource for health care professionals
A recent online survey assessing readership of articles with publication enhancements found PLS to be the most useful when compared to infographics and videos.

Patients prefer graphical formats and medium-complexity text for PLS
Research looking at different formats of PLS highlights that audience preferences should be considered when developing PLS for peer-reviewed publications.

PLS can increase the readership of research articles
Research from a pilot study shows that open-access articles that included a text-only PLS were downloaded significantly more than those without a PLS.

PLS can increase understanding of research findings for lay audiences
When compared to scientific abstracts, PLS can improve understanding, are seen as more comprehensible, cause more positive emotions, and increase the likelihood of people accessing the original article.